2.1 OCULAR MICROMETER
Introduction:
An ocular micrometer is a glass disk that attaches to a microscope
eyepiece. An ocular micrometer has a ruler that allows the user to
measure the size of magnified objects. The distance between the marks on
the ruler depends upon the degree of magnification. The ruler on a
typical ocular micrometer has between 50 to 100 individual marks, is 2
mm long and has a distance of 0.01 mm between marks.
| Microscope Eyepiece |
| Ocular Micrometer |
We can used different type of the magnifications to observe the sample. Firstly, we need to calibrate ocular micrometer before used it to observe the cells. it is because the ocular micrometer is inserted inside the ocular lens, it will not change size when the objectives are changed. Therefore, each objective lens must be calibrated separately.
- To measure cells using a microscope.
Results:

Calibration Scale Calibration:400x:5 x 0.01 = 0.050.05/ 20 = 0.0025 mm1000x:5 x 0.01 = 0.05mm0.05/ 47 = 0.0011 mm
- Lactobacillus sp :
 |
| 400x magnification
2 division x 0.0025 mm = 0.005 mm
|
 |
| 1000x magnification
5 division x 0.0011 mm = 0.0055 mm
|
- Yeast :
 |
| 400x magnification
5 division x 0.0025 mm = 0.0125 mm
|
 |
1000x magnification
7 division x 0.0011 mm = 0.0077 mm
|
Discussion:
- The first step is to correctly line up the stage and ocular micrometer (Make sure two are line up completely paralleled) .Would calculate accurate magnification ration from error margin.
- The image below shows a stage micrometer NOB1 (10μm pitch and 1mm/100 divisions) and an ocular micrometer S11 (100μm pitch and 10mm/100 divisions), with an objective lens magnification ratio at 20.
- If magnification rate is correct, 10 pichs of stage micrometer should be equal to20 pichs (2000μm) of ocular micrometer (=[1 pitch of ocular micrometer is 10μm] x 10 pichs x [Objective lens x20] ). Instead, in below diagram, 10 pichs of stage micrometer shows equals to 21 divisions of ocular micrometer, therefore it can be calculated that magnification of objective lens is x21.
- To achieve the calibrated measurement of the sample, multiply the measured value of the sample by the labeled magnification of the objective lens divided by the actual magnification ratio. (Ex. Calibrated measurement of sample = measurement of sample × labeled magnification ratio ÷ actual magnification ratio) (Calibration ratio = 20/21 = 0.95)

Conclusion:
This experiment has studied the correct way to calibrate ocular micrometer. The Ocular micrometer has a ruler those allows the user to measure the size
of magnified objects but it has no units on them without numbers. A special slides which contains scales also used
to place the objects being observed. This has to be done because the marks on the ocular
micrometer are different distances apart depending on the magnification used on
the microscope. It must be calibrated for each objective.This show how to calculate the scale using stage scale and
ocular eyepiece. The small particles such as
microorganisms or cell can be measure and the size can be compared.
t
- academic.evergreen.edu/curricular/fcb/wk2calibration.doc
- http://www.ruf.rice.edu/~bioslabs/methods/microscopy/measuring.html
- http://www.ehow.com/how_5019336_use-ocular-micrometer.html
- http://www.ehow.com/how_12103441_calculate-calibration-using-stage-micrometer.html
- http://en.wikipedia.org/wiki/Ocular_micrometer
- http://www.mecanusa.com/microscope/micrometer/micrometer-use_en.html
2.1 NEUBAUER CHAMBER
Introduction:
 These
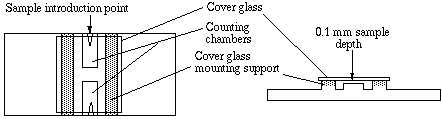
chambers are the finest quality, optically ground, and polished milled
glass chambers available. The chamber is diamond etched and has a double
improved Neubauer Ruling, which has a worldwide reputation in hospitals
and laboratories for unmatched reliability, meeting the most demanding
of standards. The standard Hausser blood counting chambers are one
piece construction (measuring 75mmx32mmx4.5mm) ensuring long term durability
and absolute accuracy in measurement and count.
These
chambers are the finest quality, optically ground, and polished milled
glass chambers available. The chamber is diamond etched and has a double
improved Neubauer Ruling, which has a worldwide reputation in hospitals
and laboratories for unmatched reliability, meeting the most demanding
of standards. The standard Hausser blood counting chambers are one
piece construction (measuring 75mmx32mmx4.5mm) ensuring long term durability
and absolute accuracy in measurement and count.  We offer both the standard (incline)
and the new “V-Load” counting hambers for different charging
methods. The tolerances are ( 2% of the volume). Cell Depth: 0.100
mm ( +/-2%); Volume: 0.1 Microliter Ruling; Pattern: Improved Neubauer,
1/400 Square mm Rulings cover 9 square millimeters. Boundary lines
of the Neubauer rulings are the center lines of the groups of three.
(These are indicated in the illustration ). The central square millimeter
is ruled into 25 groups of 16 small squares, each group separated by
triple lines, the middle one of which is the boundary. The ruled surface
is 0.10 mm below the cover glass, so that the volume over each of 16.
We offer both the standard (incline)
and the new “V-Load” counting hambers for different charging
methods. The tolerances are ( 2% of the volume). Cell Depth: 0.100
mm ( +/-2%); Volume: 0.1 Microliter Ruling; Pattern: Improved Neubauer,
1/400 Square mm Rulings cover 9 square millimeters. Boundary lines
of the Neubauer rulings are the center lines of the groups of three.
(These are indicated in the illustration ). The central square millimeter
is ruled into 25 groups of 16 small squares, each group separated by
triple lines, the middle one of which is the boundary. The ruled surface
is 0.10 mm below the cover glass, so that the volume over each of 16.
Objectives:
To count cells using microscope.
Results:
Results:
 |
| Yeast, Magnification: 400x |
sum of the
cell 10 box = 642
average =
642/10 = 64.2
volume box
(16 box) = 0.2 mm x 0.2 mmx 0.1 mm
= 4 x 10^-3 mm
= 4 x 10^-6 cm^3
so that, 64.2 cell in
4 x 10 ^-6 ml
1 ml =
642/ 4 x 10 ^-6 = 1.61 x 10^8 cell/ml
Discussion:
During this experiment we must take consistency in filling the chamber with the cell. The consistency in filling technique is paramount to successful counts.
To prepare the counting chamber the
mirror-like polished surface is carefully cleaned
with lens paper. The coverslip is also cleaned.
Coverslips for counting chambers are specially
made and are thicker than those for conventional
microscopy, since they must be heavy enough to
overcome the surface tension of a drop of liquid.
The coverslip is placed over the counting surface
prior to putting on the cell suspension. The suspension
is introduced into one of the V-shaped wells with
a pasteur or other type of pipet. The area under
the coverslip fills by capillary action. Enough
liquid should be introduced so that the mirrored
surface is just covered. The charged counting chamber
is then placed on the microscope stage and the
counting grid is brought into focus at low power.
Conclusion:
For microbiology, cell culture, and
many applications that require use of suspensions
of cells it is necessary to determine cell concentration.
One can often determine cell density of a suspension
spectrophotometrically, however that form of determination
does not allow an assessment of cell viability,
nor can one distinguish cell types.
A device used for determining the number of cells per unit volume of a suspension is called a counting chamber. The most widely used type of chamber is called a hemocytometer, since it was originally designed for performing blood cell counts. So that, the cells are counted by hemocytometer. The concentration of yeast is 161000000 cells per mL.
A device used for determining the number of cells per unit volume of a suspension is called a counting chamber. The most widely used type of chamber is called a hemocytometer, since it was originally designed for performing blood cell counts. So that, the cells are counted by hemocytometer. The concentration of yeast is 161000000 cells per mL.
- http://b110-wiki.dkfz.de/signaling/wiki/display/rnaiwiki/Counting+cells+with+haemocytometer+%28Neubauer+chamber%29
- http://sfiles.crg.es/protocols/cellculture/img/neubauer.jpg/view
- http://www.emsdiasum.com/microscopy/products/magnifier/counting.aspx
- http://people.oregonstate.edu/~weisv/Protocols/Symbiodinium/Cell%20Counts.pdf
- http://www.ruf.rice.edu/~bioslabs/methods/microscopy/cellcounting.html





No comments:
Post a Comment